From the Electrostatics
75. Polarization of dielectrics. Dielectric permittivity
Polarization of polar dielectrics.
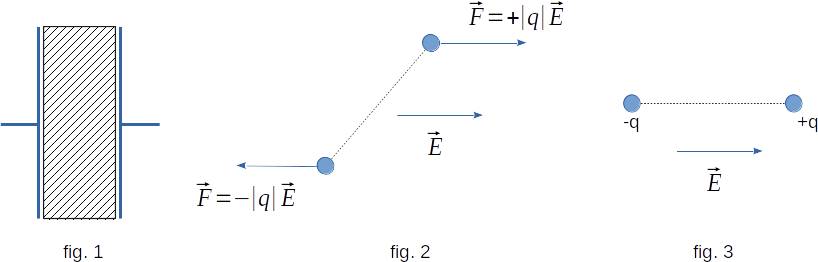
Let the polar dielectric be located between two parallel metal plates (fig. 1). If the plates are not charged and, therefore, the field strength between the plates is equal to zero, the dipoles of molecules are oriented chaotically. As a result, at all dielectric points positive and negative charges of dipoles of different molecules on average compensate each other. The dielectric does not create an electric field.So what happens in the dielectric when the plates are given the same magnitude and opposite sign charges? If the dimensions of the plates are much larger than the distance between them, an electric field will occur which, far from the edges of the plates, can be considered uniform. From the side of this field on the molecule, which is a dipole, will act two forces equal in magnitude and opposite in the direction (fig. 2). They will create a moment of force which tends to turn the dipole so that its axis is directed in the power line of the field (fig. 3).
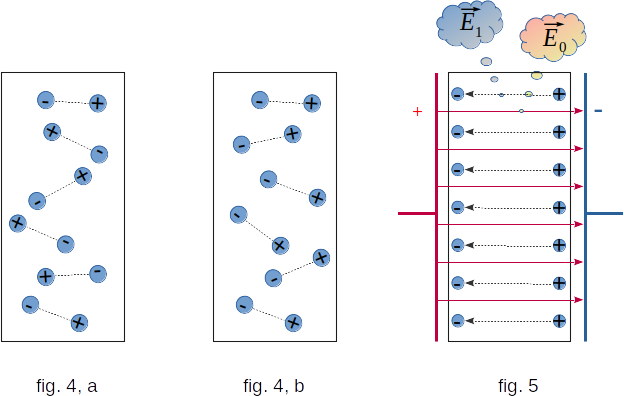
Full direct orientation of the dipole, however, is prevented by a heat movement that contributes to the chaotic orientation of the dipole (fig. 4, a). As a result, full orientation can only be achieved in very strong fields. If the field is not too strong, a state with predominant dipoles orientation along the field will be created. This means that on average, the number of dipoles oriented along the field is greater than against the field (fig. 4, b). The figure shows that due to the predominant dipoles orientation along the field, a positively charged plate will have negative dipoles, while a negatively charged plate will have positive dipoles.
As a result, a bonded charge with a certain density of \(\sigma{^{\,'}}\) occurs on the dielectric surface. Inside the dielectric, positive and negative dipole charges compensate each other and the average bound electric charge is still zero.
Polarization of non-polar dielectrics.
The non-polar dielectric is also polarized in the electric field. Under the action of the field, the positive and negative charges of the molecule shift to the opposite sides and the centers of distribution of positive and negative charges no longer coincide, as in the case of a polar molecule. Such deformed molecules can be considered as electric dipoles, whose axes are directed along the field (fig. 3). Bonded charges appear on the dielectric surfaces adjacent to the charged plates, as in the polarization of the polar dielectric.The process of generating bundled charges in the dielectric is called polarization.
Dielectric permittivity.
This bonded surface charge creates an electric field \(E_1\) directed in the dielectric against the outer field \(E_0\), which creates charges on the plates (fig. 5). This weakens the field inside the dielectric.To characterise the electrical properties of the dielectric, a special value called dielectric permittivity is introduced.
The dielectric permittivity of the medium is a physical value that indicates how many times the strength of the electric field \(E\) inside a uniform dielectric is less than the strength of the field \(E_0\) in a vacuum.
By denoting the dielectric permittivity through \(\varepsilon\), we have