From the Electrostatics
74. Dielectrics in an electrostatic field. Two kinds of insulators
The isolator, or dielectric, consists of generally neutral atoms or molecules. In dielectrics, unlike conductors, electric charges (more precisely, electrically charged particles: electrons and nuclei) in the neutral atom are connected with each other and cannot, like the free charges of a conductor, move under the influence of the field over the entire volume of the substance. Therefore, the charges in the dielectric are called bound.
The difference in structure between conductors and dielectrics leads to the fact that they behave differently in the electrostatic field.
An electrostatic field can exist within a dielectric and the dielectric has an effect on it.
The interaction force of charged bodies in a uniform dielectric, as you know, is weakened by \(\varepsilon\) times (\(\varepsilon\) is the dielectric permittivity of the medium). This is because the strength of the electric field according to the equations \((8-10)\), \((8-12)\), \((8-18)\) decreases in the dielectric by \(\varepsilon\) times. But why does the field strength change in the dielectric?
There can only be one answer. The dielectric himself, while remaining neutral, creates an electric field directed against the field created by charged bodies. After all, according to the principle of superposition, the strength of the electric field is always equal to the sum of the strengths of the fields created at a given point by all charged particles.
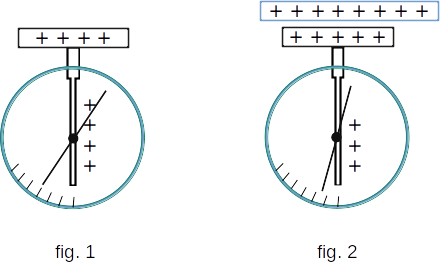
With simple experiment, you can make sure that an uncharged dielectric can create an electric field. In figure 1 you can see a charged electrometer with a metal plank at the end of the rod. If you bring a charged dielectric, such as thick glass, to the plank, the arrow of the electrometer will approach the rod (fig. 2). This can only happen if the dielectric is placed in the electric field of the charged plank himself creates the electric field. This field affects the charge distribution in the rod of the electrometer, reducing the charge of the arrow and rod and increasing the charge of the plank accordingly.
Electrical properties of neutral atoms and molecules.
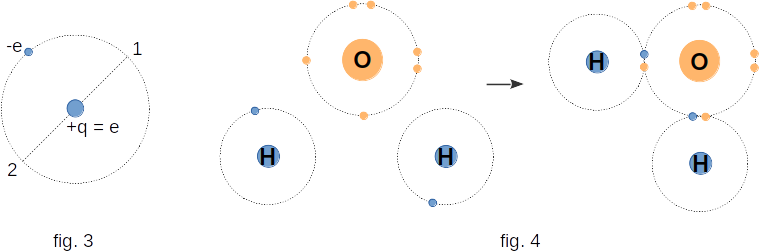
To understand how an uncharged dielectric creates an electric field, one must first become familiar with the electrical properties of neutral atoms and molecules.Atoms and molecules consist of positively charged particles - nuclei and negatively charged particles - electrons. Figure 3 shows the scheme of the simplest atom - the hydrogen atom. Positive charge of the atom, the charge of its nucleus, is concentrated in the center of the atom. The electron moves in the atom at high speed. One rotation around the nucleus it makes in a very short time about \(10^{-15}\) seconds. Therefore, for example, in \(10^{-9}\) seconds it will have time to make a million rotations and, therefore, a million times to visit points 1 and 2, located symmetrically with respect to the nucleus. This gives grounds to believe that on average in time the center of negative charge distribution falls in the middle of the atom, i.e. coincides with a positively charged nucleus.
However, this is not always the case. Figure 4 shows the arrangement of atoms in a water molecule. The hydrogen atom has one valence electron in its outer shell. The oxygen atom in the outer shell has two valence electrons. When a water molecule is formed from two hydrogen atoms their only valence electrons are captured by the oxygen atom. Both neutral atoms become a system of two ions with charges of opposite signs.
Positive and negative charges are now symmetrically distributed over the volume of the molecule: the center of distribution of the positive charge falls on the hydrogen ion, and the negative charge - on the oxygen ion.
Electric dipole.
At a large distance from the molecule of water it can be roughly considered as a set of two point charges equal in module and opposite in sign, located at some distance \(l\) from each other (fig. 5). Such a neutral system of charges as a whole is called an electric dipole.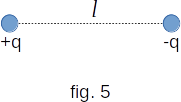
Two types of dielectrics.
We conclude that dielectrics can be divided into two types:polar, consisting of molecules, in which the distribution centers of positive and negative charges do not coincide, and
non-polar, consisting of atoms or molecules that have the same positive and negative charge distribution centers.
Polar dielectrics include alcohols, water, etc.; non-polar dielectrics include inert gases, oxygen, hydrogen, benzene, polyethylene, etc..
Insulators in physics are usually called dielectrics, from the Greek "di" - through and the English electric (the term "dielectrics" refers to substances through which are transmitted electromagnetic interactions).Ion - electrically charged atom (group of atoms).
The electrons located on an external unfinished energy level are called valence electrons. Valence is the ability of an atom to attach this or that number of other atoms to form a chemical bond.