From the Electrostatics
65. The basic law of electrostatics. The Coulomb Law
Finally, lets begin to study the quantitative laws of electromagnetic interaction. First of all let's consider the simplest case when electric charges are in a state of equilibrium, i.e. at rest. Section of electrodynamics, dedicated to the study - resting electric charges, is called electrostatics. The basic law of electrostatics is the law of interaction of two stationary point charged bodies or particles. This law was experimentally established by the French physicist Coulomb in 1785 and named after him.
Point charged bodies don't exist. But if the distance between bodies is much more than their sizes then such bodies can be considered point bodies. Naturally to expect, and experiment it confirms, that the sizes and the form of the charged bodies at the given conditions essentially do not influence on interaction between them. Remember that the law of universal gravitation is also formulated for point bodies.
The interaction force of the charged bodies depends not only on the size of the charges and the distance between them, but also on the properties of the medium between the charged bodies. For the moment, lets consider that the interaction takes place in a vacuum. However, experiment shows that air has very little influence on the interaction force: it turns out to be almost the same as in vacuum.
The Coulomb's law is the basic (fundamental) physical law and can only be established by experiments. It does not follow from any other laws of nature.
Setting by the Coulomb the quantity of the interaction force between electric charges was made easier by the fact that these forces are relatively large. Therefore, it was not necessary to use particularly sensitive equipment, as in the verification of the law of gravity in the earth conditions. With the help of torsional balances it was possible to establish how stationary charged bodies interact with each other. A torsional balance is simply a glass stick attached to a thin elastic wire (fig. 1). On one end of the stick is attached a small metal ball, and on the other - a counterweight. Another metal ball is fixed to the balance wall stationary.
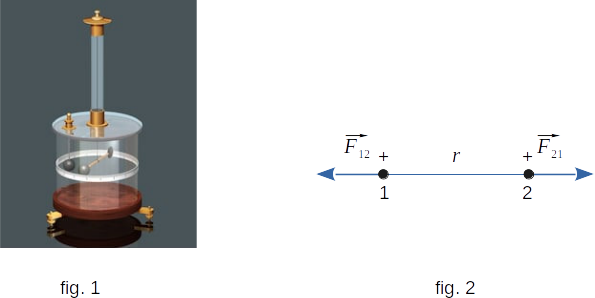
The interaction force of the balls can be measured by the angle of twisting the wire and the dependence of this force on the distance between the charges and their size. We were able to measure the force and distance at that time. The only difficulty was with the charge, for measuring which there were no even units.
Coulomb acted simply and wittily. He changed the size of the charge of one of the balls in 2, 4, etc. times, connecting it to the same uncharged ball. The charge was evenly distributed among the balls, which reduced the size of the studied charge in a certain respect. The change of force when the charge was changed was determined experimentally.
Coulomb's experiments led to the establishment of a law that remarkably reminded the law of universal gravitation. The force of interaction of point stationary electrically charged bodies in vacuum is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
If we denote the value of the charges \(q_1\) and \(q_2\), and the distance between them \(r\), then the Coulomb's law can be written in the following form
\( F ~= ~k\frac{q_1 \,q_2}{r^2} \) (8-1)
where \(k\) is the coefficient of proportionality
(in the SI system, Coulomb's constant is \(k_e \approx 9 \times 10^9 ~N \cdot m^2 \cdot C^{−2}\)).
As long as no units of all values are entered, the experiment gives only proportionality of force to the values of the charges and inverse proportionality to the square of the distance. The equality can only be established when all the quantities \(F, r, q_1\) and \(q_2\) have been measured. You already know the units for measuring \(r\) and \(F\), and the unit of charge has yet to be entered. Only after selecting the units to measure the charge the coefficient \(k\) will be a strictly defined value.
So far, nothing has been said about the direction of interaction forces between charges. Experiments show that Coulomb forces, i.e. the interaction forces of two point charged bodies, act along a line connecting these bodies (fig. 2). Such forces are called central.
Knowledge of the law Coulomb - this is the first concrete step in the study of the property of electric charge and in finding out the meaning of the concept of electric charge. The presence of electric charge in elementary particles or bodies means that at rest they interact with each other according to the law of the Coulomb.