From the interaction of atoms and molecules in substance
Mutual transformations of liquids and gases
46. Liquefaction of gases
Any gas can be converted into a liquid by simple compression, unless its temperature is below critical. Therefore, the division of substances into liquids and gases is largely conditional. The substances that we are used to considering as gases simply have very low critical temperatures and therefore cannot be in a liquid state at temperatures close to room temperature. On the contrary, the substances we classify as liquids have high critical temperatures (see table in \(\S 43\)).
All gases that make up the air (except carbon dioxide) have low critical temperatures. Their liquefaction therefore requires deep cooling.
There are many types of machines for producing liquid gases, in particular liquid air.
In modern industrial plants, significant cooling and liquefaction of gases is achieved by expansion under thermal insulation conditions.
These machines are called detander (turboexpanders). Expanding gas performs the work by moving the piston (piston expanders) or rotating the turbine (turbine expanders Kapitsa) using its internal energy and therefore cools down.
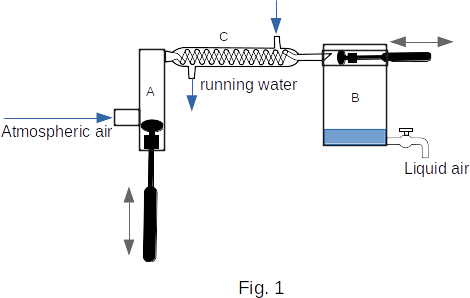
Figure 1 shows a simplified scheme of a piston detander (expander). The atmospheric air enters into compressor A, where it is compressed to a pressure of several tens of atmospheres. The gas heated by compression is cooled down in the heat exchanger C by running water and arrives in the cylinder of a detander B. Here the gas performs the work, pushing the piston, and cools down so strongly that it condenses into the liquid.
Liquid air has a boiling point of \(-193^0C\) at atmospheric pressure. And various gases, of which it consists, boil at different temperatures. The lowest boiling point has helium, neon, nitrogen and argon. Oxygen has a slightly higher boiling temperature. Using the difference in boiling point, it is possible to extract gases from the liquid air. Gases with a low boiling temperature evaporate most rapidly, for example, nitrogen evaporates before oxygen. This is the basis for the industrial production of inert gases, which exist in small quantities in the air. Inert gases are widely used to fill electric incandescent and gaslight bulbs (see \(\S 109\)).
Liquid gases are stored in special containers called Dewar containers. The Dewar container is designed in the same way as a regular thermos. It has double glass walls, with air being pumped out of the space between them. This reduces the thermal conductivity of the container. The inner wall is made shiny (silver plated) to reduce the heat generated by the radiation. In a good Dewar container, liquid air is stored for a few days.
Liquefied gases are widely used in practice. Nitrogen is used for the production of ammonia, an important chemical raw material. The use of inert gases has already been mentioned. The most important is liquid oxygen. It is used in metal processing (oxygen blowing), and for medical purposes. The engines of the first space rockets were powered by liquid oxygen.