From the Molecular-kinetic theory
24. Basics principles of molecular-kinetic theory
Perhaps the most important thing that we have learned about the world around us is that all bodies are made up from very small particles that are in continuous motion. The particles are attracted to each other at short distances and are repulsed by close approaching.
Molecular-kinetic theory of substance structure is based on three positions, each of which is now proved by experiment: the substance consists from particles; these particles move chaotically; the particles interact with each other.
The properties and behavior of bodies, ranging from rarefied gases from the upper atmosphere to solids on Earth, as well as super-dense nuclei of planets and stars, are ultimately determined by the movement of particles that interact with each other, from which consisted all bodies: molecules, atoms or even smaller formations - elementary particles.
Proof of the existence of molecules. Size of molecules.
The assumption that all bodies consist of separate particles, and, hence, no one body can be divided into as small parts as you like, was expressed by ancient thinkers Democritus and others 2500 years before our time. Qualitative explanation of many phenomena with the help of molecular theory was given in the XVIII century by Swiss mathematician and physicist Daniel Bernoulli in his book Hydrodynamica (1738). However, it was only the end of the 19th century and the beginning of the 20th century that the existence of molecules and atoms was indisputably proved. The thing is, the atoms are very small. They are not only invisible in the eye, but also in the microscope. That's why all the bodies seem solid to us.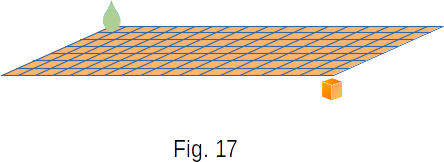
The first strict proof of the existence of atoms and molecules was obtained in chemistry. Now there are many proofs. To be sure of the existence of molecules, it is necessary to determine their size.
One of the simplest ways to estimate the size of molecules is as follows.
It is known that it is impossible to spray a drop of Olive oil with the volume of \(1 mm^3 \) on the surface of water more than \(0.6 \, m^2 \) area. It can be assumed that when the oil spreads over the maximum area, it forms a layer only in one molecule. The thickness of this layer is easy to calculate and estimate the size of the molecule of olive oil.
Let's cut a cube of \(1 \, mm^3 \) into square layers, \(1 \, mm^2 \) each, so that they could cover an area of \(0.6 \, m^2 \) (Fig. 17). The number of such layers will be
\(\frac{0.6 \, m^2}{0.000001 \, m^2} = 6 \times 10^5 \)
From here we obtain that the thickness of the oil layer, and therefore the particle size of Olive oil is
\(\frac{0.1 \, cm}{6 \cdot 10^5} \approx 1.7 \times 10^{-7} cm\)
With such small molecules, the number of them in any macroscopic body is enormous. Thus, there are about \(3 \times 10^{22} \) of them in a drop of water with the mass of \( 1g \). The body is called macroscopic if it consists of a very large number of molecules.
The size of molecules is so small that an attempt to imagine these sizes clearly exceeds the possibilities of our imagination. What can the number \(2.3 \times 10^{-8} cm \) tell us - the approximate size of a hydrogen molecule? As always in such cases, comparisons help the imagination. If the pen you write with is magnified enough to take it out from Earth to the Moon, the diameter of the hydrogen molecule with the same magnification will be the size of the pen.
Movement of molecules.
The most obvious proof of the movement of molecules can be obtained by observing the tiniest insoluble particles in the water in the microscope. These particles make a chaotic movement called Brownian motion as a result of countless irregular pushes of surrounding molecules. Later on we will learn more about Brownian motion.Proving the existence of significant forces of interaction between atoms or molecules is not difficult at all if you try to break a wooden stick. But it consists of molecules, so these forces help solid bodies to maintain the shape and volume.
Motion laws.
The basic principles of molecular-kinetic theory, which were discussed, allow us to explain qualitatively the basic properties of bodies and features of heat phenomena. But to create a quantitative theory it is necessary to know the laws of molecular motion. We are familiar with the laws of Newton's mechanics. They describe the motion of bodies containing a large number of molecules - macroscopic bodies. It is not obvious in advance that the movement of individual molecules should obey the same laws.Suppose, however, that the motion of molecules is subject to the laws of Newton's mechanics. Where the consequences of the theory arising from this assumption do not contradict experiment, this assumption can be considered valid. It does prove to be true, but not in all cases.
For example, the motion of atoms or molecules in gases is subject to the laws of classical mechanics. However, the internal motion of particles in atoms cannot be described using the laws of Newton. Here it is necessary to use the laws of Quantum mechanics.
The application of Newton's mechanical laws to describe the motion of atoms and molecules in liquids and solids depends on temperature. For example, at sufficiently high temperatures, the motion of solid body atoms relative to the nodes of the crystal structure is well described by the laws of Newton, and at low temperatures it is necessary to use Quantum mechanics
Further on we will not try to build a quantitative molecular theory of solids and will limit ourselves to gases only. Therefore we can consider that motion of molecules is subject to the laws of Newton mechanics.