From the interaction of atoms and molecules in substance
Mutual transformations of liquids and gases
39. Specific heat of vaporization
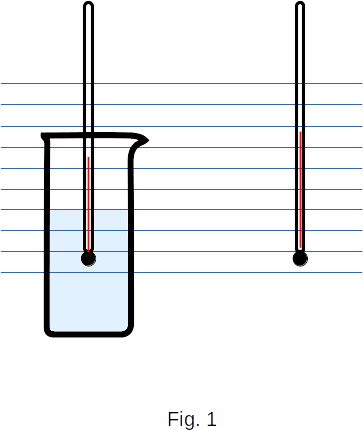
To keep the temperature of the evaporating liquid constant, it is necessary to bring heat to it. Of course, the heat itself can also be transmitted from surrounding bodies. For example, the water in the glass evaporates, but the temperature of the water, which is slightly lower than the ambient temperature (Fig. 1), remains constant. Heat is transferred from the air to the water until all the water has evaporated.
The quantity of heat required to convert a liquid into vapour at a constant temperature related to a unit of mass \((1kg~\) or \(~1g)\) is called the specific heat of vaporization.
What does this heat take up? First of all, to work against the attraction forces between the molecules when individual molecules leave the liquid (output work). In other words, heat is used to increase the potential energy of the molecules when they leave the liquid.
Another part of the vaporization heat is used to work against external pressure forces when the volume of the substance increases. The volume of vapour is greater than the volume of liquid it is formed from. Evaporation is accompanied by an increase in the volume of the substance. This part of the evaporation heat is usually a few percent from the total at room temperature.
As the temperature rises, the specific heat of vaporization decreases, because as the temperature rises, the kinetic energy molecules increase. Therefore, the bonding forces (molecular attraction forces) are already struggling to hold fast-moving liquid molecules against each other. To break the bond of one of the molecules with the rest of the liquid, less additional energy is enough.
For example, the specific heat vaporization of water changes with increasing temperature as follows
with \(0^0C ~- 595 ~cal/g\)
with \(50^0C ~- 568 ~cal/g\)
with \(100^0C ~- 539 ~cal/g\)
with \(200^0C ~- 468 ~cal/g\)