From the Electrical current in different environments
116. Electrical conductivity of semiconductors with impurities
In the previous paragraph, we examined the conductivity mechanism of ideal semiconductors that do not contain any impurities. Conductivity under these conditions is called own conductivity.
Own conductivity of semiconductors is usually small, as there are few free electrons. For example, in an element of germanium in one cubic centimeter at room temperature the number of free electrons \(~ n_e \,= \,3 \cdot 10^{13} \,cm^{-3}\). At the same time, the number of germanium atoms in one cubic centimetre is about \(~ 10^{23}\). Thus, the number of free electrons is about one ten billionth of the total number of atoms.
Own conductivity of semiconductors is similar to conductivity of electrolytes. In both cases free charge carriers arise as a result of heat motion. Therefore, both semiconductors and electrolytes have increased conductivity with increasing temperature.
Essential feature of semiconductors consists in that in them at presence of impurity along with own conductivity occurs additional impurity conductivity. By changing the concentration of impurities, the number of charge carriers of a negative or positive sign can be significantly changed. Thanks to that it is possible to create semiconductors with predominant concentration of either negative or positive charge carriers. This feature of semiconductors opens up great opportunities for their practical application.
Donor impurities.
It turns out that in the presence of impurities, such as arsenic atoms, even at very low concentrations, the number of free electrons increases many times. This is happening for the following reason. Arsenic atoms have five valence electrons. Four of them are involved in creating a chemical bond with surrounding atoms, such as silicon atoms. The fifth valence electron is weakly bound to the atom. It easily leaves the atom and becomes free (fig. 1).
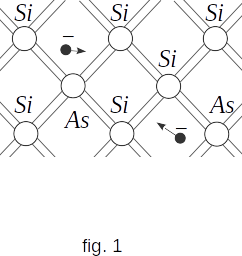
When one ten millionth of arsenic atoms is added, the free electron concentration becomes \(~ 10^{16} \,cm^{-3}\). This is one thousand times more free electron concentrations of pure semiconductor.
Impurities that easily give away electrons and, therefore, increase the number of free electrons are called donor (giving) impurities.
As semiconductors having a donor impurity possess a greater number of electrons (in comparison with the number of holes), they are called n-type semiconductors (from the word negative). In semiconductors of n-type electrons are the main carriers of charge, and the holes are non-main.
Acceptor impurities.
If an element of indium whose atoms are trivalent is used as an impurity, the nature of conductivity of a semiconductor changes. Now, for the formation of normal paired electron bonds with the neighbors of the indium atom there is not enough electron. The result is a hole in the atom. The number of holes in the crystal will be equal to the number of impurity atoms.
These kinds of impurities are called acceptors.
In the presence of an electric field, the holes move around the field and hole conductivity occurs. Semiconductors with a predominance of hole conductivity over the electronic are called p-type semiconductors (from the word positive). The main charge carriers in p-type semiconductors are holes.