From the Electrical current in different environments
105. Electric current in liquids
Liquids, like solids, can be dielectrics, semiconductors and conductors. Distilled water, for example, is one of the dielectrics.
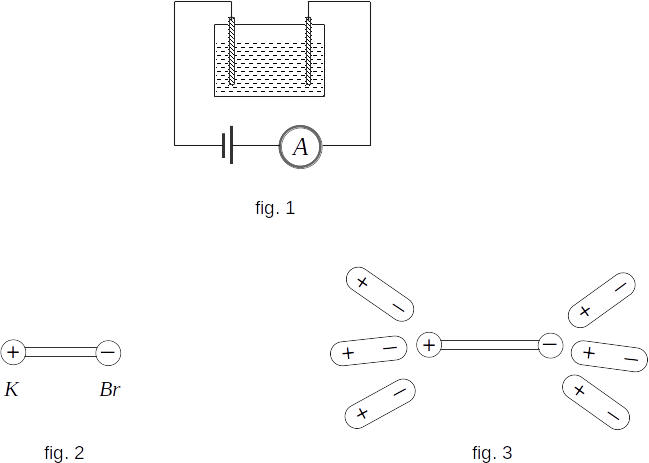
Distilled water has very low conductivity, which is easy to see. To do this, put down two electrodes in a glass water jar and assemble the circuit as shown in figure 1.The electric current in the circuit is almost zero. However, if you add even a small amount of salt to the water, the current in the circuit will appear.
Therefore, the salt solution, unlike distilled water, is electrically conductive.
Salt solutions that have electrical conductivity, as mentioned, are called electrolytes. Electrolytes are also solutions of alkalis, acids and molten salts.
Let's find out the mechanism of conductivity of electrolytes by the example of potassium bromide solution \(KBr\).
The interaction of bromine and potassium atoms in a molecule of potassium bromide can be simplified as the interaction of two ions: positively charged ion \(K^+\) and negatively charged ion \(Br^-\) (fig. 2). The point is that the only valence electron in potassium is weakly bound to the atom. At formation of the \(KBr ~\) molecule this electron passes to bromine turning it into negative \(Br^-\) ion. According to this, the \(KBr ~\) molecule can be schematically represented as a dipole (fig. 3).
When potassium bromide salt dissolves in water, \(KBr ~\) molecules are surrounded by water molecules, which are also dipoles. In the electric field created by the \(KBr ~\) molecule, the water molecules are oriented as shown in figure 3. In doing so, they stretch the \(KBr ~\) molecule so that a slight shaking of it in collision with other molecules destroys it. Part of \(KBr ~\) molecules disintegrates - it dissociates into \(K^+\) and \(Br^-\) ions. This process is called electrolytic dissociation.
The degree of dissociation, i.e. the proportion of dissolved substance molecules that disintegrate into ions, depends on the temperature, solution concentration and dielectric permittivity \(~ \varepsilon ~\) of solvent. With increasing temperature, the degree of dissociation increases and, consequently, the concentration of positively and negatively charged ions increases.
Ions of different signs at a meeting can again be united in neutral molecules - recombine.
At invariable conditions in the solution is established dynamic equilibrium, in which the number of molecules that decay into a unit of time on the ions is equal to the number of pairs of ions, which at the same time again reunite into neutral molecules.
Thus, the carriers of charge in electrolytes are positively and negatively charged ions.
If the electrolyte vessel is connected to the electrical circuit, the negative ions will move to the positive electrode, the anode, and the positive ions to the negative electrode, the cathode. This will result in an electric current. Since charge transfer in electrolytes is carried out by ions, conductivity of electrolytes is called ionic.
Liquids can also have electronic conductivity. Liquid metals, for example, have this conductivity.
When electric current passes through an electrolyte, for example, copper sulfate solution \(CuSO_4\), the following process occurs. Positive \(Cu^{++}\) ions when in contact with the cathode get the missing electrons and are emitted on the cathode in the form of neutral atoms. Negative \(~ SO^{--}_4 \) ions give off extra electrons when they come into contact with the anode. The electrons appearing on the anode pass through an external circuit to the cathode and there they are connected to the positive ions.
The substances often extracted at the electrodes enter into chemical reactions which are not directly connected with the passage of electric current. This complicates the phenomenon of electric current in liquids. In particular, a reaction of \(~ SO_4 \,+ \,Cu \,= \,CuSO_4 ~\) occurs on the copper anode, accompanied by the dissolution of the anode.
The process of extraction on the electrodes of substances that are part of the electrolyte is called electrolysis, as already mentioned.
Electrolysis is widely used in technology for various purposes.
By electrolysis, for example, the surfaces of one metal are covered with a thin layer of another (nickel plating, chromium plating, copper plating, etc.). This strong coating protects the surface against corrosion.
If measures are taken to ensure that the electrolytic coating is well peeled off from the surface on which the metal was deposited (this is achieved, for example, by applying graphite to the surface), you can get a copy of the relief surface.
In the printing industry, such copies (matrixes) are obtained from a set of letters, deposition of a sufficiently thick layer of iron on it. Matrixes serve as a mould for casting, allowing to reproduce a set of letters in the required number of copies. If earlier the circulation of the book was limited by number of prints which can be received from one set of letters (at printing the set of letters is erased), application of electrolysis in printing, allows to increase circulation of the book practically unlimitedly.
Depositing metal on the long cylinder, it is possible to receive pipes without a seam.
With help of electrolysis do clearing of metals from impurity. So, the uncleaned copper received from ore is cast in the form of thick plates which then are placed in a tub as anodes. During electrolysis, copper on the anode will dissolve, impurities containing valuable and rare metals, fall to the bottom, and the cathode will settle pure copper.
The electrolysis process is used to produce aluminium from a bauxite melt. This way of producing aluminium made it the cheapest and perhaps the most common metal in technology and everyday life.