From the Electrical current in different environments
107. Electric current in gases
The conditions under which electric current can flow through gases are very diverse. Electric current can pass through rarefied gases, such as in daylight bulbs and advertising tubes. When lightning strikes and in an electric arc, current flows through the air at atmospheric pressure.
Conductivity of gases.
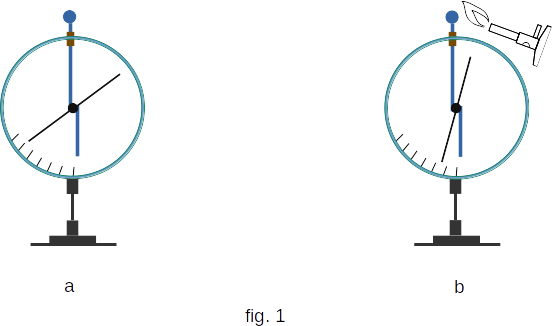
The conductivity mechanism of gases is similar to the mechanism of electrolytes. But there are also significant differences between them. One of the main differences is that the carriers of charge in gases are not only ions, but also electrons, ie, gas as if combines the conductivity of metals - electronic conductivity and conductivity of electrolytes - ionic conductivity.How does conductivity arise in the air? At room temperature, gases, including air, are very bad conductors. This is easy to see by observing the arrows of the charged electrometer (fig. 1, a). The charge of the electrometer remains unchanged for a long time. Due to a number of reasons (heating, radiation of radioactive substances, X-rays) air conductivity increases. The easiest way to detect an increase in the conductivity of the gas when it is heated. To do this, bring a candle or burner to the electrometer ball (fig. 1, b). Note that the electrometer is quickly discharged. It shows that in the air at heating there are charges, neutralizing a charge of an electrometer.
Let's consider the reasons of change of electric properties of gases at the specified influences.
Gas ionization.
Under normal conditions, gases are almost entirely made up of neutral atoms or molecules and are therefore dielectric. As a result of heating or radiation, some atoms are ionised (disintegrated into positively charged ions and electrons). Negative ions can also form in the gas and they are caused by the connection of the electron to the neutral atom.The simplest method of ionization of gases at heating is explained by the fact that as the molecules are heated, they move faster. In this case, some molecules begin to move so fast that some of them disintegrate in collisions, turning into ions. The higher the temperature, the more ions are formed.
Recombination.
If the ionizer ceases to work, you will notice that the charged electrometer will retain its charge again. This shows that when the ionizer stops working, the gas ceases to be a conductor.This is due to the fact that when the electron and the positively-charged ion meet, for example, they can form a neutral atom again. Schematically this is shown in figure 2. This process is called recombination (reunification) of charged particles. Therefore, when the ionizer ceases to work, the charged particles disappear due to recombination and the gas becomes a dielectric again.
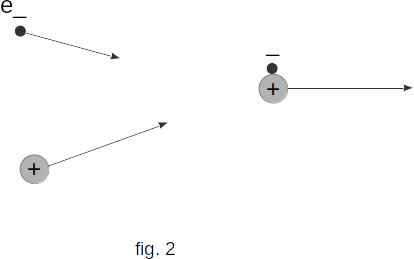
If the ionizer action is constant, a dynamic equilibrium is established in which the number of newly formed pairs of charged particles equals the number of pairs that disappear due to recombination.