From the Electrical current in different environments
103. Electric current in metals
Experimental evidence that the conductivity of metals is a result of the movement of free electrons, was given in experiments carried out by Richard C. Tolman and T. Dale Stewart in 1916. (Tolman, R. C.; Stewart, T. D. (1916). "The electromotive force produced by the acceleration of metals")
The scheme of these experiments is as follows. A wire is spooled onto a coil, the ends of which are connected to two metal discs isolated from each other (fig. 1). A galvanometer is connected to the ends of disks by means of sliding contacts.
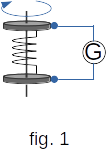
The coil is driven into a quick rotation and then abruptly stopped. Based on the model according to which the electric current in metals is created by the movement of free electrons, we should expect that after a abrupt stop of the coil free electrons will move for some time on inertia and thus create a short-term electric current until they slow down by collisions with ions.
Observations have shown that it is true that after stopping the coil, electrical current does flow in the circuit for a short time. Its direction indicates that it is created by the movement of negatively charged particles. The quantity of the charge carried in this case is proportional to the ratio of the charge of the particles generating the electric current to their mass, i.e. \(e/m\). Therefore, by measuring the quantity of the charge passing through the galvanometer during the whole period of existence of electric current in the circuit, it was possible to determine the ratio \(e/m\). It turned out to be equal to \(1.8 \cdot 10^{11} q/kg\). This value agrees well with the value of the ratio e/m for electrons, found from other experiments.