From the Direct electric current
101. Polarization of galvanic elements
The first galvanic element, the Volta element, consisted of copper and zinc electrodes, like Daniel's, but both electrodes were submerged into one electrolyte, a sulphuric acid solution. The Volta element gives a direct electric current only for a short time after the circuit is closed. This current then decreases rapidly as the element's EMF drops.
Here's the thing. In the \(~ H_2SO_4 ~\) aqueous solution, there are positive ions of hydrogen. When the element is working, they are deposited on a copper electrode. As a result, some time after the circuit is closed, this electrode is covered with a thin layer of hydrogen. Instead of the copper electrode, a "hydrogen" electrode is produced. This process is called element polarization.
The potential jump at the border of hydrogen - electrolyte is smaller than at the border of copper - electrolyte. Therefore EMF decreases. In other words, the polarization of the element leads to the appearance of additional polarization EMF, which has a sign opposite to that of the element EMF. This causes the element EMF to decrease. At the same time the allocation of hydrogen strongly increases the internal resistance of the element, because the hydrogen film on the copper electrode has a high resistance.
For the stable work of the element it is necessary to prevent the accumulation of hydrogen on the positive electrode of the element. Depolarization of the positive electrode is said to be necessary. In Daniel's element this is achieved by using two electrolytes, selected so that the chemical composition of the electrodes does not change during the operation of the element. The copper electrode is in copper sulfate solution, and in a closed circuit on the positive electrode it is not hydrogen but copper that is deposited.
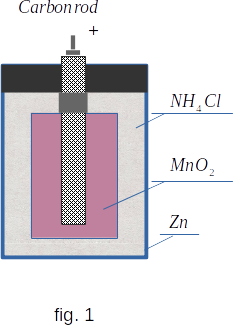
The most common is the chemical depolarization method, which consists in oxidation of hydrogen and its transformation into water. Exactly on this principle based depolarisation in the most currently used element, the Leklanshe element. The negative electrode of the Leklanshe element is also zinc. The positive electrode consists of a carbon rod surrounded by a mixture of a strong oxidant - manganese peroxide \(~ Mn\,O_2 ~\) and graphite to increase electrical conductivity (fig. 1). The electrolyte is a solution of ammonium chloride - \(~NH_4Cl.~\) In "dry" elements instead of liquid electrolyte thick starchy mass impregnated with ammonia is used. Gaseous hydrogen is not formed, as there is a reaction
\(Mn\,O_2 \,+ \,2H \,= \,Mn\,O \,+ \,H_{2}\,0\)
which results in manganese oxide and water. Emf element Leklanshe approx. \(~ 1.4v.\)
The phenomenon of electrode polarization is used in batteries.