From the Heat phenomena
5. Gas laws. Boyle-Mariotte law
The quantitative dependence of pressure, temperature and volume on each other is the simplest form for gases, especially for gases where pressure is not very high (does not significantly exceed the pressure in one atmosphere\(^1\)) and the temperature is not too low.
1 atmosphere unit of pressure is equal to pressure of \(760mm\) high mercury column. \(1atm \approx {103 000 {N \over mm^2}} \approx {10^5 {N \over mm^2}}\)
The main property of gases, which distinguishes them from liquids, is the ability of gases to expand unlimitedly. No matter how large the volume of the container is, the gas always puts pressure on all its walls. Experience has shown that if the gas temperature does not change, its pressure increases with decreasing volume. It is easy to see this by squeezing the volleyball or soccer balloon, which is weakly inflated with your hands: when you squeeze the balloon, the air pressure in it increases.
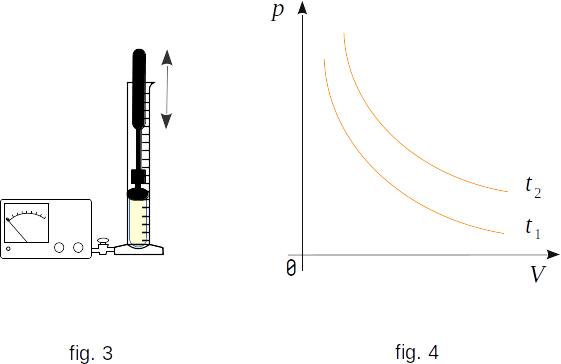
The pressure dependence on volume can be measured with the device shown in figure 3. The hermetic bulb is connected to the manometer that registers the pressure inside the bulb. When the plunger is pressed, the volume inside the bulb changes. The volume can be seen with a ruler on the bulb. Changing the volume and observing the value of pressure, it is easy to make sure that for a given mass of gas how many times the volume decreases, as many times the pressure increases. The pressure a given mass of gas multiplied by its volume equals a constant if the gas temperature does not change.
Suppose the gas pressure was \(p_1\) at first and the volume \(V_1\), then, after compression, the pressure became \(p_2\) and the volume \(V_2\). Then
\(p_1\,V_1 \,= \,p_2\,V_2\)
or
\(p\,V \,= \,const\) if \(t \,= \,const\) (1-1)
This dependence was published back in the 17th century, almost simultaneously by two scientists: the Anglo-Irish scientist Robert Boyle and the French physicist Edme Mariotte. It was therefore called the Boyle–Mariotte law.
The process of changing the state of the system at a constant temperature is called isothermal.
Graphically, the dependence of gas pressure on volume is represented by curves called isotherms (fig. 4).
Different temperatures correspond to different isotherms, in Figure 4 \(t_2 > t_1\). According to the Boyle-Mariotte law the gas isotherma express inversely proportional relation between pressure and volume \(p = \frac{1}{V} \). In mathematics, this kind of curve is called a hyperbola.
The isothermal process can only occur when it has a good heat exchange between the gas container and the thermostat - system that has some means of regulating the temperature. An atmosphere can also be used as a thermostat if the air temperature remains the same for a long time.