From the Heat phenomena
3. Temperature. Thermal equilibrium
In geometry, you have one basic value - length. The other values: area and volume are derivatives. In kinematics one more value is added - time. Velocity, acceleration are derivatives. In dynamics the main additional value is mass. In the theory of heat phenomena, the one new basic value to be entered is temperature.
In addition to sensitive receivers that respond to touch, pressure and pain, there are receivers in the human skin that respond to external influences with a feeling of warmth and cold. With this in mind, all bodies can be arranged in a row in terms of their ability to make us feel warm or cold. The reason for this ability of bodies to affect the senses differently can be explained by various degrees of body heat - temperature. This is only a qualitative determination of the temperature, which does not include any indication of how the temperature is to be measured. You met with it in the first book. To find the exact definition of temperature it is necessary to define the concept of thermal equilibrium.
To find out your body temperature, hold the thermometer under your arm for 5-8 minutes. During this time, the mercury in the thermometer gradually heats up and the level rises. After the set time has elapsed, the thermometer will stop heating and you will determine the temperature along the length of the mercury column. The same thing happens when you measure the temperature of any body with any thermometer. The thermometer readings will remain the same for as long as the body temperature is the same.
Another example. Put an ice cube in the glass of water and cover it with a solid lid. The ice cube will melt and the water will cool down. When the ice melts, the water will start to heat up, and once it has reached ambient temperature, there will be no change inside the glass of water.
From these and other simple observations, we can conclude that there is a very general pattern of heat phenomena. Under unchanged external conditions, for the period of time the body spontaneously passes to the state of thermal equilibrium with surrounding bodies. In this state such values as volume and pressure remain unchanged. Thermal equilibrium of the body can be preserved for as long as it takes.
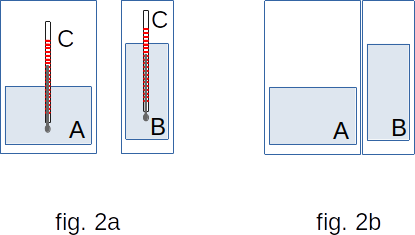
It is essential that if any body A ( for example, water in one cup, Fig. 2a) is in thermal equilibrium with another body C (thermometer), and body B (water in the other cup) is also in equilibrium with body C, then bodies A and B are also in thermal equilibrium. If the glasses are brought into contact (Fig. 2b), no changes will occur to the water inside them. That's why we can compare the state of thermal equilibrium of bodies without bringing them into direct contact and define the concept of temperature.
Zeroth Law of Thermodynamics
We say that two bodies A and B have the same temperature if each of them is in thermal equilibrium with the third body C. This body could be a thermometer. Conversely, if the bodies have the same temperature, it is possible to assert, without bringing them into direct thermal contact, that they are in a state of thermal equilibrium.
When contact is made between two bodies with different temperatures, their temperatures are leveled out. Just as pressure equality in two containers with the same gases means that when the containers are connected by a tube, the gas will not move from one container to another, temperature equality between the two bodies means that when a thermal contact is established between them, there will be no heat transfer from one body to another.
Temperature is the only magnitude that has the same value in any part of the system during thermal equilibrium. The volume of the different parts of the system with the hard partitions and the pressures inside them may vary. So, if you bring the ball from the street to the room, after a while the air temperature in the ball and the room will level off. The air pressure in the ball will still be bigger than the room pressure.
Temperature measurement devices - thermometers usually use the property of different liquids to change their volume when heated. The liquid must be such that different temperatures and volumes must correspond to different temperatures. This condition is not fulfilled for water. Indeed, when water is heated from 0 to 4°C, its volume decreases and then begins to increase.
It's not just the volume of the fluid that changes as the temperature changes. Gas pressure, electrical resistance of conductors and etc. also changes. All these phenomena can be used to measure temperature.