From the Surface tension in liquids
49. Molecular picture of the surface layer
Now let's move on to a more detailed acquaintance with surface tension.
The molecules at the interface and the two environments are in different conditions than the molecules at the depth of the liquid. The molecule at the depth of the liquid is surrounded from all sides by neighboring molecules. The molecule at the liquid surface gets the influence of other molecules only from the liquid side. The vapor density at temperatures far from critical is much lower than the liquid density (Fig. 1). Consequently, the forces of interaction of the molecule at the surface with the molecules of vapour can be neglected.
Remember that molecules are attracted to each other at a distance of the order of several molecular radii and repelled at very close distances. Attraction forces acting on a surface layer molecule from all other molecules give an equal, downward force. However, from the side of neighboring molecules, the repulsion forces act on the given molecule. Thanks to this, the molecule is in equilibrium. It is true that any molecule participates in heat movement. But for liquid molecules, this movement is reduced to fluctuations near some equilibrium positions. And from time to time molecules change their equilibrium positions. In place of the molecule that has gone deep into the liquid, another molecule comes, etc.
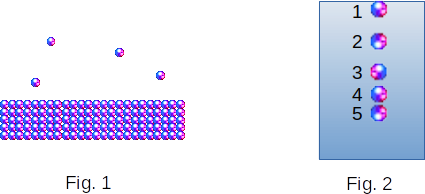
As a result of the forces of attraction and repulsion, the density of liquid in the surface layer is lower than inside. In fact, molecule \(1\) (Fig. 2) is affected by the repulsion force of molecule \(2\) and the force of attraction of all other molecules \(3, 4, 5\) and so on. Molecule \(2\) is affected by the same force of attraction on the side of molecules in the depth and the same force of repulsion on the side of molecule \(3\). But, in addition, there is also the force of repulsion on the side of molecule \(1\). It brings the molecules \(2-3\) closer. As a result, the distance \(1-2\) is on average greater than the distance \(2-3\). The distance \(2-3\) is greater than the distance \(3-4\), etc., until the proximity of molecules to the surface stops affecting it. Thus, the molecules of the surface layer are on average at greater distances from each other than the molecules inside the liquid. Therefore, the increase in the liquid surface is accompanied by the appearance of new areas of the rarefied surface layer. And it requires performing work against the forces of attraction between the molecules.