From the Magnetic properties of the substance
144. Explanation of para- and diamagnetism
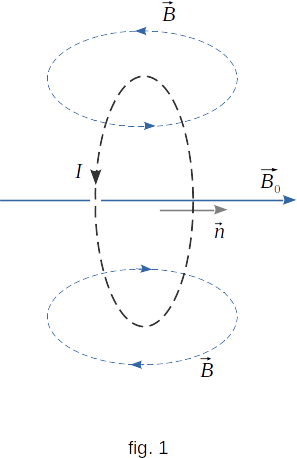
The difference between para- and diamagnetic properties of the substance is explained by the difference in magnetic properties of their atoms. In atoms and molecules of paramagnetic bodies moving electrons create a magnetic field in the surrounding space. These atoms can be considered approximately as small closed ring currents (fig. 1).
In atoms and molecules of diamagnetic substances the currents created by the motion of electrons have such a configuration that the magnetic fields created by them mutually compensate each other.
When paramagnetics is placed in an external field, individual elementary currents tend to orient themselves in the direction of the field. The normals toward ring currents tend to be oriented along the magnetizing field \(\overrightarrow{B}{_0}\). The elementary current fields therefore increase the external field. The chaotic thermal motion of atoms prevents the correct orientation of currents and the higher the temperature, the smaller percentage of elementary currents are oriented in the direction of the magnetic field. As a result, the higher the temperature, the weaker the magnetism of the substance. When the magnetizing field disappears, the thermal movement completely destroys the orientation of currents and the substance is demagnetized.
Why do diamagnetics also detect magnetic properties, even though their atoms without a magnetizing field do not create a magnetic field? When a magnetic field appears (or when a substance moves into an area where this field already exists) in atoms or molecules, inductive currents are generated in the same way that in the transformer's secondary winding a current is induced when the magnetic field permeates this winding. In contrast to the transformer, however, the induced current in the atom does not decay even after the EMF of the induction has disappeared, since there is no resistance to the motion of electrons in the atoms. Diamagnetism entirely comes from the electromagnetic induction phenomenon. According to Lents law, the direction of induction current is such that the magnetic field created by current is directed against the magnetizing field (fig. 2). It should be understood as follows: when the magnetic field appears the vortex electric field appears as well. This vortex field causes electrons to twist around magnetic lines \(\overrightarrow{B}{_0}\). Additional rotation is imposed on previously existing motion of electrons. This rotation results in a magnetic field directed against the magnetising field \(\overrightarrow{B}{_0}\).
When the magnetic field \(\overrightarrow{B}{_0}\) disappears, the induction currents disappear and the diamagnetic is demagnetized.
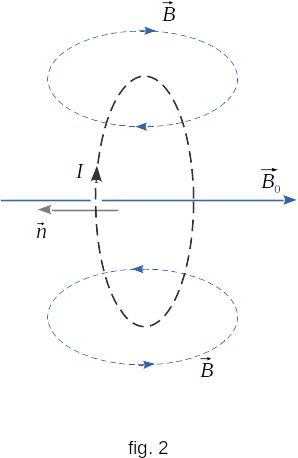
It is clear from the above that diamagnetism is inherent in all bodies without exception, since all atoms placed in the magnetic field generate additional induced current. But only those substances appear to have diamagnetism, whose atoms or molecules do not create a magnetic field by themselves.
In paramagnetic, and even more so in ferromagnetic bodies, atoms have their own "natural" magnetic properties and when elementary currents are oriented in atoms, a strong magnetic field is created which suppresses a weak diamagnetic effect.
It should also be noted that the diamagnetic properties of the bodies, in contrast to paramagnetic bodies, do not depend on temperature. Thermal motion of atoms in general does not disturb the orientation of induced currents inside atoms.