From the Solid bodies and their transformation into liquids
55. Amorphous bodies
Along with crystalline solids there also exist amorphous bodies. In contrast to crystals, amorphous bodies have no strict order in the arrangement of atoms. Only the nearest neighboring atoms are in approximate order. But there is no strict repeatability in all directions of the same element of structure, which is typical for crystals, in amorphous bodies.
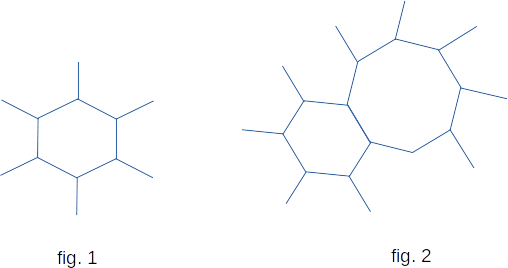
Often, the same body may be in both crystalline and amorphous states. For example, quartz (\(SiO_2\)) can be in both crystalline and amorphous form (silica, or silicon dioxide). The crystalline form of quartz can be schematically represented as a grid of regular hexagons (fig. 1). The amorphous structure also has the form of a lattice, but not correct. Along with hexagons there are pentagons and heptagons in the lattice (fig. 2).
All amorphous bodies are isotropic, their physical properties are the same in all directions. To amorphous bodies belong glass, many plastics, gums, rosin, sugar lollipop.
Under external influences, amorphous bodies find both elastic properties like solids and fluidity like liquids. Under short-term influences (hit) they behave like a solid body and are split into pieces under a strong hit. But on very long exposures, amorphous bodies flow. For example, a piece of gum gradually spreads over a hard surface. This is due to the fact that atoms or molecules of amorphous bodies, like molecules of liquid, have a certain time of sedentary life - the time of oscillation near the equilibrium position. But unlike liquids, this time is very long. In this respect, amorphous bodies are close to crystalline, because the jump of atoms from one equilibrium position to another occurs rarely.
At low temperatures, amorphous bodies are more like solid bodies in their properties. They have almost no fluidity. But as temperatures rise, amorphous bodies gradually soften and their properties are more and more like liquids. This is because as the temperature rises, atoms gradually jump from one position of equilibrium to another. There is no definite melting point for amorphous bodies.